Renewable chemicals and technology
For the transition from fossil to renewable feedstocks, a better understanding of heterogeneously catalyzed reactions aiming at the rational design of next generation catalysts and cutting-edge reactor configurations is crucial. With an original focus on petrochemical reactions today the focus has been extended towards sustainable and even renewable feedstocks.
In this research theme, emphasis is on the development of fundamental so-called microkinetic models together with dedicated ab initio calculations and elaborate catalyst characterization to enable this extension. The individual phenomena investigated range from the chemical kinetics, over mass and heat transport phenomena and phase/thermodynamic effects, to non-ideal reactor hydrodynamics. They are all integrated in a comprehensive reactor model allowing to adequately extrapolate the laboratory scale observations up to the industrial scale. Significant efforts are spent in the automation of model construction and complex mixtures are dealt with in many of the investigated topics.
Our key research questions:
- How to ensure an adequate rational catalyst design in combination with high-throughput kinetics?
- How to efficiently deal with key aspects, such as the presence of O-atoms, in the extension of modeling methodologies from conventional to alternative, renewable feeds?
- What are the critical properties to control the activity and selectivity in liquid phase reactions?
- Can we reliably extrapolate laboratory-scale kinetics to commercial scale operation?
The following topics are part of the Renewable chemicals and technology research theme:
Multi-scale design of reactors for catalyzed processes
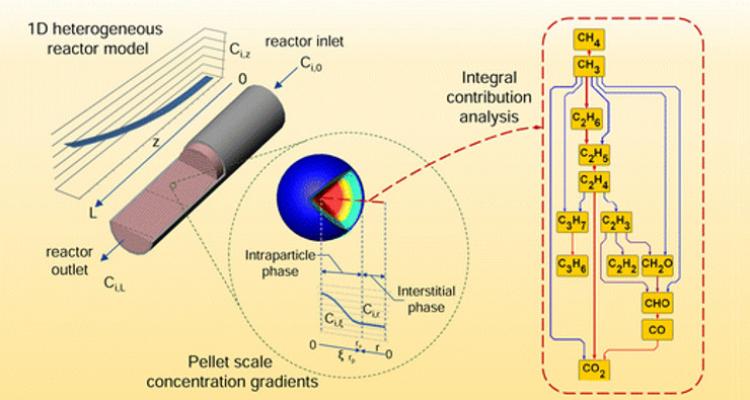
A chemical reactor is a multi-scale apparatus with occurring phenomena, ranging from the atomic scale of bond breaking and formation, over mass and heat transport at the micro/meso scale, up to the hydrodynamics at the largest scale. The ultimate challenge is to account for the elementary phenomena at these different scales to identify the dominant ones, depending on the condition used. Pushing the boundaries towards the use of microkinetic models will allow to tune the stability, activity and selectivity of the catalytic performance in these reactors.
Using hydrotreatment to upgrade conventional and renewable oil fractions and achieve cleaner emissions
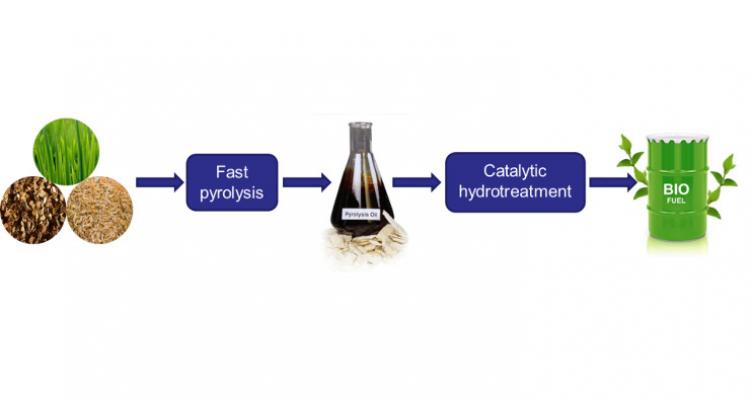
Upgrading the quality of both conventional and renewable oil fractions is frequently achieved by means of a treatment under hydrogen at elevated pressure. This reduces the unsaturation level of the feed and also leads to hetero-atom removal. Another important advantage is that cleaner emissions are achieved when using the oil products as fuel.
Metal-based catalysis for small molecule activation and selective conversion
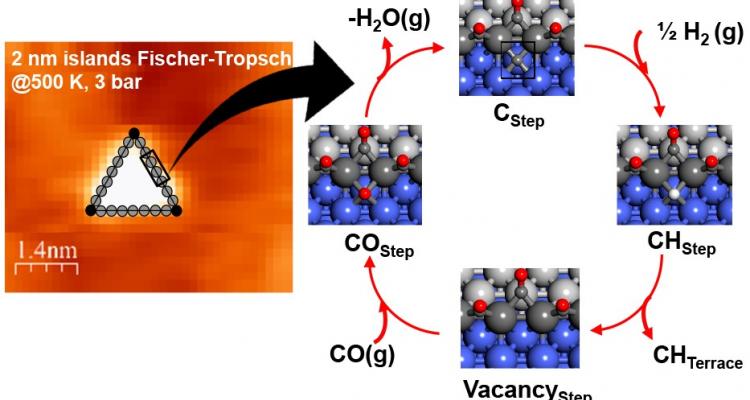
The activation and selective conversion of small molecules (e.g., CH4, CO, CO2, N2) over metal-based catalysts remains an important challenge for catalyst design and optimization. At the LCT, we combine in situ characterization, modelling-guided design and intrinsic kinetic measurements to develop novel catalysts and processes for these important transformations.
Metal catalysis to tailor the conversion of renewables into added-value products
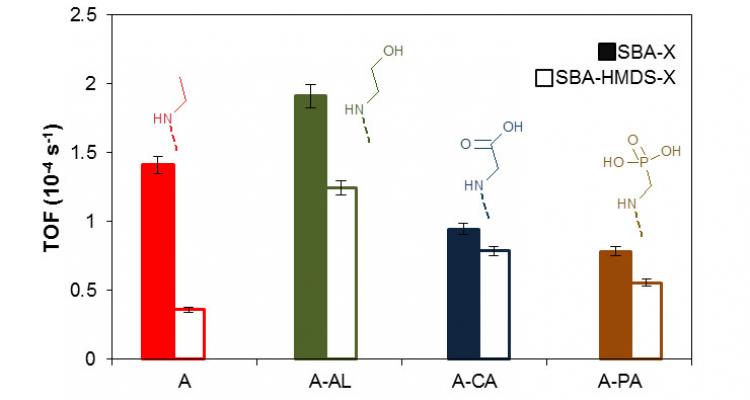
A wide range of metals can be used to efficiently catalyze the transformation of complex molecules. Activity, selectivity and stability of these catalysts are key issues to be addressed in the pursuit of optimally performing materials. Nanoparticles, alloys and specific promoting elements all open up promising opportunities to further tailor the conversion of renewables into added-value products.
Providing chemical insights to use biomass as a resource for high-value chemicals
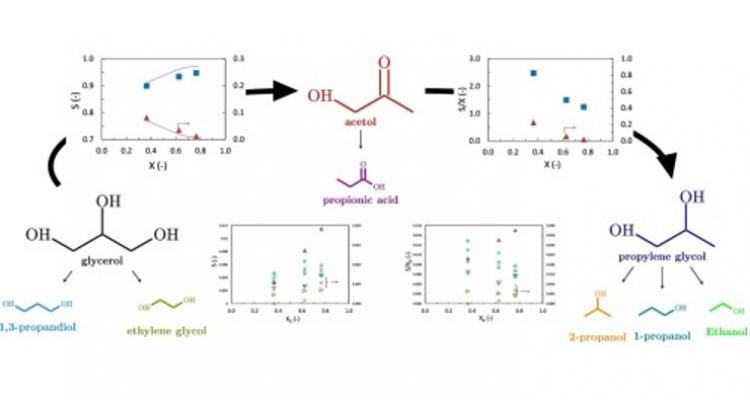
The diversity of functional groups makes biomass an interesting resource for chemicals. To be economical feasible, all biomass components need to be valorized. Experimental and theoretical studies provide the chemical insight needed to optimize the yields of high-value chemicals and upgrade lower-value products.
Designing kinetic models to optimize biomass conversion into green, next-generation fuels
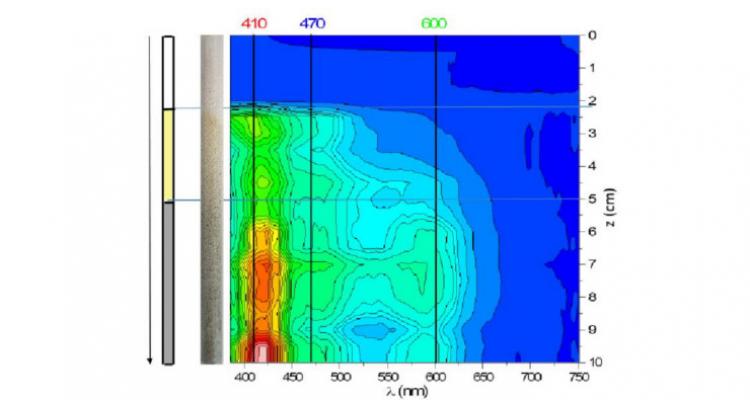
Thermal (catalytic) biomass conversion to green, next-generation fuels provides a promising means to reduce the carbon footprint and the emission of dangerous particulates. Experimental and theoretical studies lead to detailed kinetic models needed to optimize fuel production and to assess fuel performance.
Applying acid-based catalysis on renewables
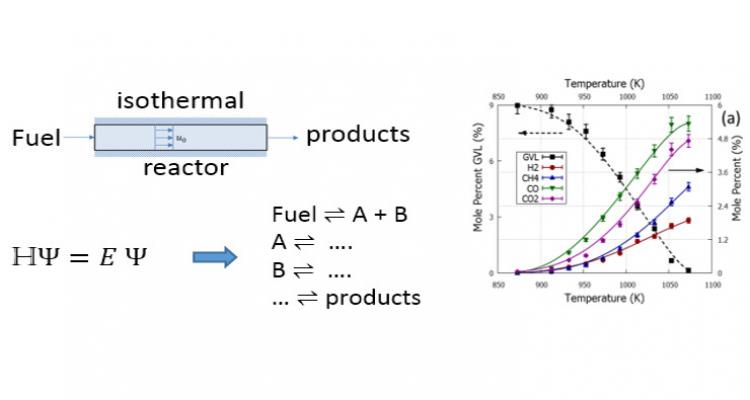
Acid catalyzed hydrocarbon conversion has a long history in refining operations and rapidly extends into the area of renewables. Complex mixtures are efficiently dealt with and - thanks to the limited number of types of elementary steps -, acid catalyzed reactions are ideally suited for fundamental kinetic modeling, such as the Single-Event MicroKinetic methodology.
Investigating heat and mass transfer in vortex units
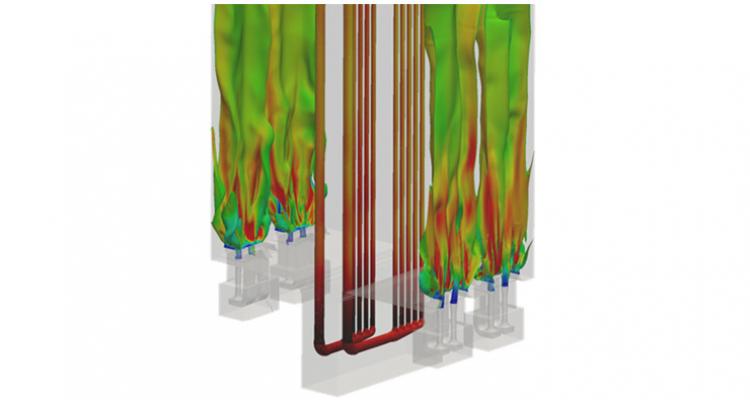
The effect of unit geometry and operational variables on the hydrodynamic performance - as well as on the reactor and powder scale heat and mass transfer -, is investigated in an adiabatic scaled-down gas-solid vortex unit. Correlations for the dimensionless groups describing the transfer rate dependencies on geometry and process conditions are developed.
Plastics chemical recycling and biomass valorization
Using a unique infrastructure for detailed characterization - such as comprehensive two-dimensional gas chromatography (GC × GC) and comprehensive LC equipment -, allows to obtain valuable new information about waste and alternative feedstocks valorisation. In combination with feedstock reconstruction, process evaluations can be executed in silico with unprecedented accuracy.
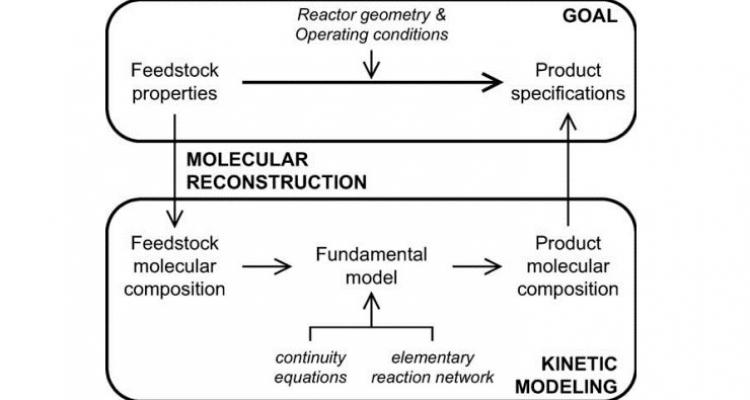
Developing an automatic chemical reaction mechanism generator to optimize computer simulations
Developing or optimizing chemical reactors based on computer simulations is becoming indispensable in the chemical industry, as this allows to circumvent the use of expensive and time consuming experiments. The development of GENESYS, an automatic chemical reaction mechanism generator which is able to build kinetic models – composed of elementary chemical reaction steps -, is an important step to improve the value and applicability of computer simulations.
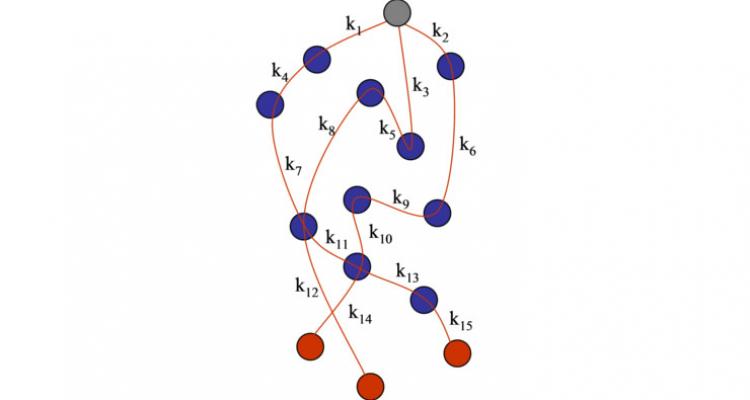
Analyzing complex reaction networks through transient techniques and steady-state kinetic studies
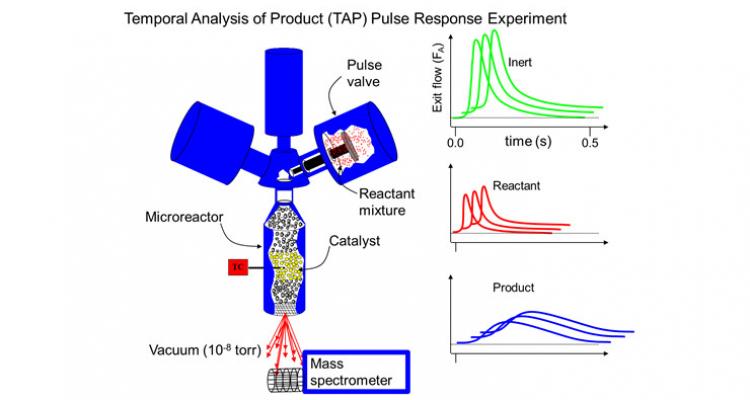
Transient techniques are particularly effective for analyzing complex reaction networks, because the rates of the various elementary steps in the catalytic cycle are not equal during transient experiments. Thus, elementary steps can be distinguished more easily, leading to a more detailed insight into the reaction kinetics. A TAP (Temporal Analysis of Products) reactor setup allows conducting a variety of transient and steady-state kinetic measurements.
Alcohol as a bio-based feedstock through optimization of the preprocessing phase

The use of alcohol, one of the prime bio-based feedstocks, requires preprocessing before it can be used as a building block in the chemical industry. The first step for the majority of processes and applications is the removal of water in catalytic dehydration. A combination of an experimental and computational approach allows to understand and optimize these catalysts.
Designing effective solutions to control the selectivity of catalysts
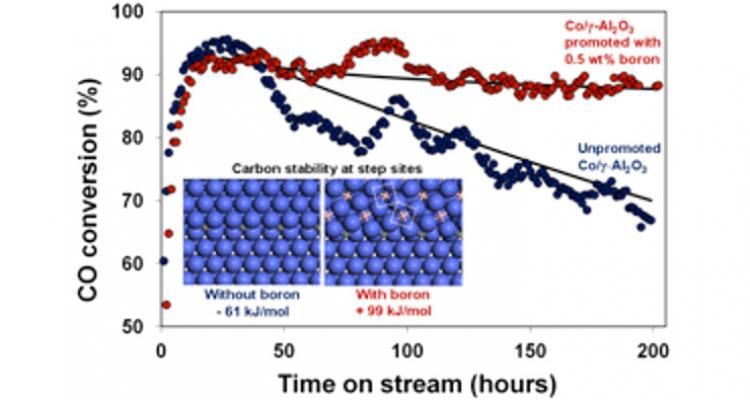
The ultimate goal for catalysis is to design catalysts with high stability, activity and selectivity. Being rather well-established for catalyst stability and activity, design principles for selectivity remain to be developed. Using first principles modeling to elucidate the catalyst structure and the nature of the active sites under reaction conditions, innovative solutions to control selectivity are sought and validated experimentally.
Designing numerical methods for chain-growth processes
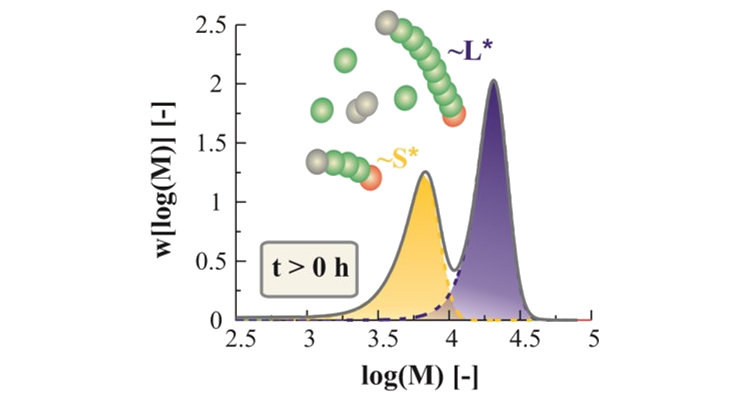
Several chemical processes include a chain or step-growth mechanism, in which species with different chain lengths are created. Deterministic as well as stochastic methods are developed in order to describe and design these chemical process into detail, including depolymerization.
Creating efficient energy use and exergy analysis
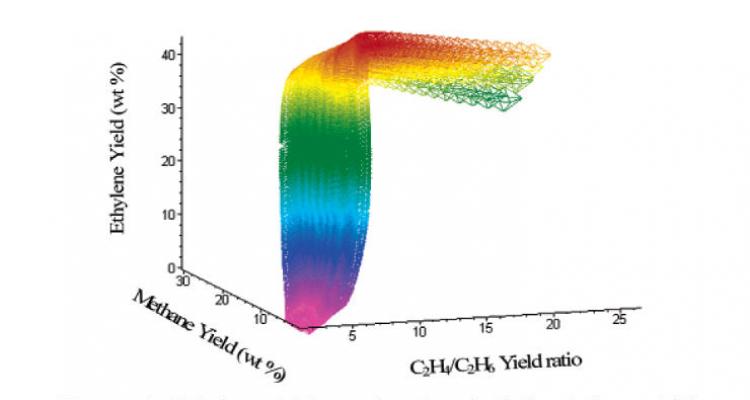
A key challenge for industrial production processes is their energy efficient operation. Here optimal conditions towards maximal performance are identified for thermal and catalytic processes including polymerization.
Biogas as a source for platform chemicals
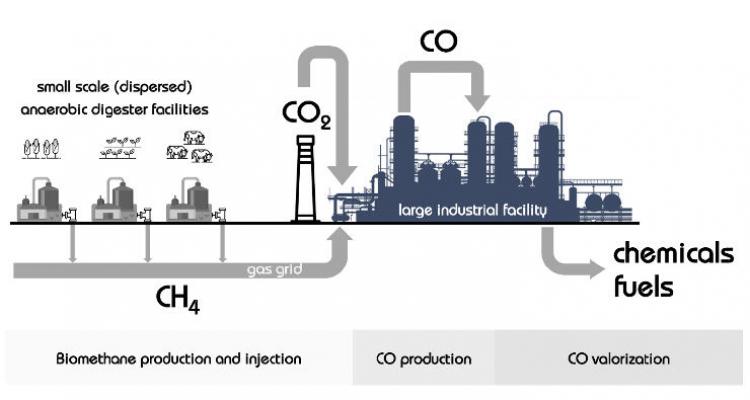
The biogas sector is rapidly growing and novel achievements lay the foundation for biogas plants as advanced bioenergy factories. Methane activation is one of the most challenging reactions to facilitate further conversion into higher molecules.
