Sulfur poisoning: Rh facilitates Ni site regeneration
(22-04-2020)
Effect of Rh in Ni-based catalysts on sulfur impurities during methane reforming, Stavros-Alexandros Theofanidis, Johannis A. Z. Pieterse, Hilde Poelman, Alessandro Longo, Maarten K. Sabbe, Mirella Virginie, Christophe Detavernier, Guy B. Marin, Vladimir V. Galvita, Applied Catalysis B: Environmental, Volume 267, 2020, p. 118691,
https://doi.org/10.1016/j.apcatb.2020.118691
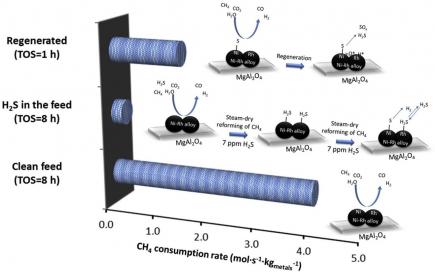
The addition of Rh, in low concentrations (<1 wt%), to Ni-based catalysts was investigated during steam-dry reforming of a gas mixture, which simulates the effluent of a biomass gasifier. Presence of H2S at ppm level had a strong impact on the reforming performance, as all the examined samples lost 90 % of activity. The catalyst with a Ni:Rh molar ratio of 41 showed the best performance in terms of both activity and stability, in presence of 7 ppm H2S as a contaminant, at 1173 K. The catalyst regeneration ability depended on the formation of a Ni-Rh alloy and hence on the Ni:Rh molar ratio. According to density functional theory calculations on the adsorption and dissociation of H2S on Ni and NiRh (111) surfaces, the Ni-Rh alloy inhibited H2S decomposition in contrast to monometallic Ni. This suggests that doping of Ni-based catalysts with a low Rh concentration provides a route to increase the sulfur resistance of the catalyst, due to inhibition of H2S dissociation and retaining H2S adsorbed in its molecular form on Rh. During regeneration with an H2S-free feed stream, the adsorbed H2S can then quickly desorb without requiring hydrogenation of S and SH intermediates.
